
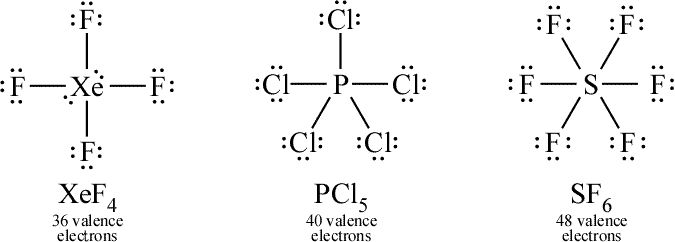
Thus species such as SF 6 are often called expanded-valence molecules A compound with more than an octet of electrons around an atom. Sulfur has an 3 s 23 p 43 d 0 electron configuration, so in principle it could accommodate more than eight valence electrons by using one or more d orbitals. To accommodate more than eight electrons, sulfur must be using not only the ns and np valence orbitals but additional orbitals as well. The octet rule is based on the fact that each valence orbital (typically, one ns and three np orbitals) can accommodate only two electrons. The third step in our procedure for writing Lewis electron structures, in which we place an electron pair between each pair of bonded atoms, requires that an atom have more than 8 electrons whenever it is bonded to more than 4 other atoms. Each fluorine atom has an octet, but the sulfur atom has 12 electrons surrounding it rather than 8. If we arrange the atoms and electrons symmetrically, we obtain a structure with six bonds to sulfur that is, it is six-coordinate. Let’s look at sulfur hexafluoride (SF 6), whose Lewis structure must accommodate a total of 48 valence electrons. Examples from the p-block elements include SF 6, a substance used by the electric power industry to insulate high-voltage lines, and the SO 4 2− and PO 4 3− ions. Such compounds are found for elements of period 3 and beyond and involve the participation of a d orbital in the bonding. The most common exception to the octet rule is a molecule or an ion with at least one atom that possesses more than an octet of electrons. Even without such a treatment, we notice that such odd electron species are extremely reactive. Molecules such as NO, NO 2, and ClO 2 require a more sophisticated treatment of bonding, which will be developed in Chapter6. With 5 + 6 = 11 valence electrons, there is no way to draw a Lewis structure that gives each atom an octet of electrons. Some important examples are nitric oxide (NO) nitrogen dioxide (NO 2), an oxidizing agent in rocket propulsion and chlorine dioxide (ClO 2), which is used in water purification plants. There are, however, a few molecules containing only p-block elements that have an odd number of electrons.
Molecules or ions containing d-block elements frequently contain an odd number of electrons, and their bonding cannot adequately be described using the simple approach we have developed so far.

Because most molecules or ions that consist of s- and p-block elements contain even numbers of electrons, their bonding can be described using a model that assigns every electron to either a bonding pair or a lone pair.


 0 kommentar(er)
0 kommentar(er)
